Health officials have urgently recalled a common daily pill taken by cancer survivors over fears some batches may not work.
UK medicines watchdog the Medicines and Healthcare Products Regulatory Agency (MHRA) stuck an alert on one batch of Tamoxifen 20mg tablets.
It is feared the drug may not dissolve into the bloodstream effectively after failing routine dissolution tests.
But the MHRA, which published the alert, said it had not yet received any complaints or reports of harm from patients who had taken the 30-pack tablets.
The recall only impacts one batch of the pills, manufactured by Wockhardt UK Limited, with the batch number HZ10030 and an expiry date of April 30, 2027.
Tamoxifen is a daily pill taken by an estimated 550,000 British breast cancer survivors. It can slash their risk of recurrence after treatment by up to 45 per cent.
It’s also offered to women with a strong family history of the disease, as it cuts their odds of ever developing it.
Dissolution tests are commonly conducted by regulators and manufacturers to check the time taken for the medication’s active ingredient to release into the body.
UK medicines watchdog the Medicines and Healthcare Products Regulatory Agency (MHRA) stuck an alert on one batch of Tamoxifen 20mg tablets
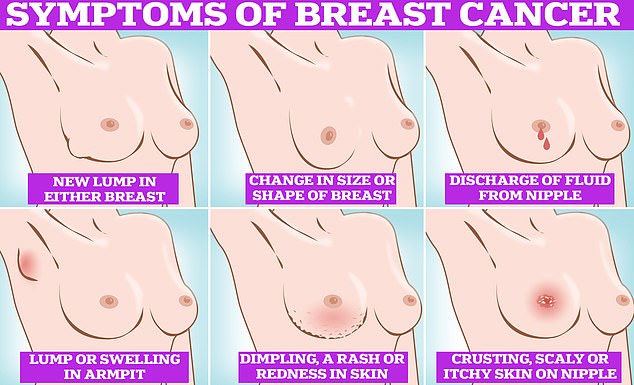
Symptoms of breast cancer to look out for include lumps and swellings, dimpling of the skin, changes in colour, discharge and a rash or crusting around the nipple
This helps predict how the drug performs inside the body.
Their effectiveness relies on the drug dissolving in the fluids of the gastrointestinal tract prior to absorption into the blood stream.
The dissolution procedure is an important test both to evaluate safety, predict efficacy and stability with respect to manufacturing and storage conditions.
In its recall, the MHRA said patients should continue to take medicines as prescribed by their healthcare professional.
Healthcare professionals such as pharmacists should stop supplying the batch immediately and quarantine all remaining stock.
But any patients who experience an adverse reaction or have questions about the recall should seek medical attention.
Adverse reactions should also be reported via the MHRA’s Yellow Card scheme.
The scheme, set up in the 1960s, allows doctors, pharmacists and patients themselves to report adverse reactions believed to be caused by prescription and over-the-counter drugs, implants and alternative medicines.
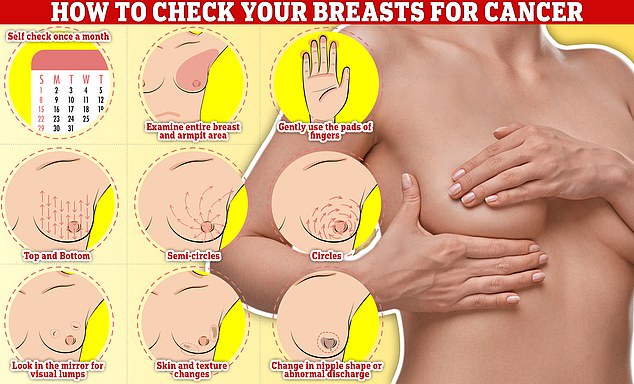
Checking your breasts should be part of your monthly routine so you notice any unusual changes. Simply rub and feel from top to bottom, in semi-circles and in a circular motion around your breast tissue to identify any abnormalities
This can lead to them being reviewed, having warnings added to the label or even being taken off the market.
Tamoxifen, which blocks the activity of the female sex hormone oestrogen in the body, started life in 1962 as a contraceptive pill.
Then, in the 1980s, trials showed that when given to breast cancer patients after surgery and other treatments such as chemotherapy and radiotherapy, it further reduced the risk of the disease coming back.
By stopping oestrogen from reaching the cancer cells, it meant tumours grew more slowly or ceased growing altogether.
Today, it is one of the most taken drugs to treat the disease, and is recommended by prescribing watchdog the National Institute for Health and Care Excellence for about 80 per cent of breast cancer patients.
And, after further studies found that it reduced the chances of breast cancer developing in the first place, in 2013 it became the first drug to be prescribed to prevent cancer.











