Thousands of women have been thrown a lifeline thanks to a ‘next generation’ drug that can destroy breast cancer tumours, months before they even grow.
The daily pill, known as camizestrant, stops cancer cells from developing, slowing the spread of the disease and delaying the need for gruelling chemotherapy.
Around seven in ten breast cancer patients in the UK have a type of the disease known as HR positive HER-2 negative breast cancer—the most common form.
Of these, around 40 per cent, totalling roughly 10-15,000 UK women every year, can develop an aggressive genetic mutation that makes their outlook incredibly bleak.
But the ‘transformational’ trial found patients given the drug camizestrant saw their risk of the cancer progressing slashed by more than half.
It was also the first worldwide study that showed blood tests, rather than scans or invasive biopsies, can pick up early signs of cancer returning.
Doctors used the test to spot changes in the cancer’s DNA—when they found signs of the harmful mutation, some patients were given camizestrant, while others stayed on their usual treatment.
Experts presenting the findings today at the American Society for Clinical Oncology conference (ASCO) in Chicago, hailed it a ‘pivotal moment in breast cancer care’ and ‘truly fundamental shift in how we approach cancer’.
The daily pill, known as camizestrant, disrupts cancer cells from developing further, slowing the spread of the disease and delaying the need for gruelling chemotherapy
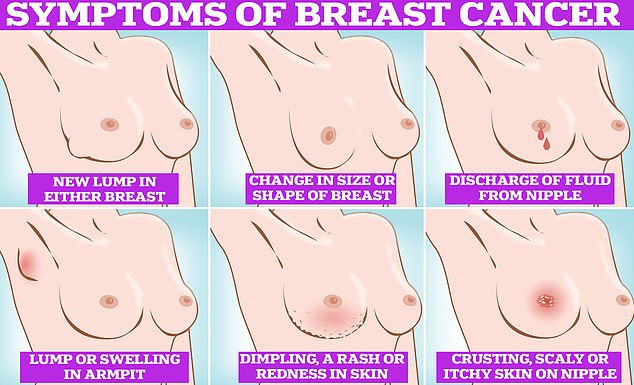
Symptoms of breast cancer to look out for include lumps and swellings, dimpling of the skin, changes in colour, discharge and a rash or crusting around the nipple
The drug is already being fast-tracked for use in the US and has been sent for approval in the UK.
Professor Nicholas Turner, an expert in molecular oncology at The Institute of Cancer Research, London and the Royal Marsden NHS Foundation Trust, who co-led the major trial, said: ‘This is a pivotal moment in breast cancer care.
‘This proactive approach also redefines how we think about drug resistance in this type of breast cancer.
‘This is a potential new treatment strategy in oncology to treat developing resistance before it causes disease progression.’
Professor Kristian Helin, chief executive of The Institute of Cancer Research, London, added: ‘The results represent more than a clinical milestone—they represent a transformational shift in how we approach precision medicine.
‘It is very exciting to see this technology being used to delay disease progression in patients and extend the benefits of treatment in patients with this type of advanced breast cancer and delay the need for chemotherapy for as long as possible.
‘These breakthroughs are helping shape personalised breast cancer treatment, allowing doctors to adjust therapies earlier and improve patient outcomes.’
In the trial, 3,325 patients HR positive HER-2 negative advanced breast cancer from 23 countries were screened for ESR1 mutations using a liquid biopsy every eight to 12 weeks.

Presenting the findings at ASCO, Susan Galbraith, executive vice president of oncology at AstraZeneca said the drug had now been given ‘breakthrough therapy designation’ by the Food and Drugs Administration in the US, helping to speed up regulatory review
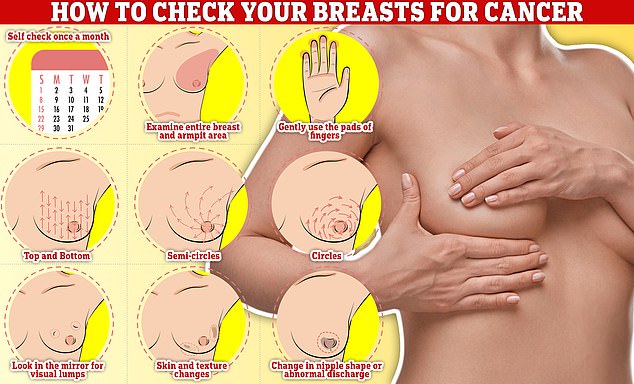
Checking your breasts should be part of your monthly routine so you notice any unusual changes. Simply rub and feel from top to bottom, in semi-circles and in a circular motion around your breast tissue to identify any abnormalities
Of these, 315 women who tested positive for an ESR1 mutation were given either AstraZeneca’s camizestrant and a medicine known as a CDK4/6 inhibitor or another hormone therapy as well as a CDK4/6 inhibitor.
Researchers found those on the camizestrant combination slashed their risk of death or the cancer progressing by 56 per cent.
The drug also kept the cancer at bay for 16 months on average compared to 9.2 months on standard treatment.
Just one per cent of patients stopped taking the drug over side effects.
Presenting the findings at ASCO, Susan Galbraith, executive vice president of oncology at AstraZeneca said the drug had now been given ‘breakthrough therapy designation’ by the Food and Drugs Administration in the US, helping to speed up regulatory review.
‘We are having ongoing discussions with regulatory authorities including the UK’, added.
Dave Fredickson, AstraZeneca’s executive vice president of oncology business unit, also said the drug demonstrated a ‘truly fundamental shift in how we approach cancer care.
‘We’re moving away from a one size fits all era and targeting cancer early.’
Meanwhile, Dr Catherine Elliott, director of research at Cancer Research UK, said: ‘This study is a clear example of how blood tests are starting to transform cancer treatment.
‘By tracking tiny traces of tumour DNA in the blood, researchers were able to spot early signs of treatment resistance and switch therapies before cancer had a chance to grow.
‘It shows how circulating tumour DNA—or ctDNA—could help doctors make smarter, more timely treatment decisions.
‘This approach could become an important part of how we personalise care for people with advanced breast cancer.’












