A woman who shed four stone on Mounjaro but suffered life-threatening side effects has warned the public to be wary of the blockbuster weight loss jabs.
Aimee Chapman, 34, from Southampton, was admitted to intensive care last summer with liver failure and a perforated oesophagus—complications doctors linked to the popular injections.
The former waitress bought the drug from a major online pharmacy in March 2024.
She hoped that weight loss would lead to he being ‘taken more seriously’ by doctors, after being diagnosed with fibromyalgia, a chronic pain condition.
Initially the medication proved successful, with Ms Chapman shedding four stone in just four months.
A few months in, her energy levels plummeted, and more troubling symptoms began to emerge.
She said: ‘I couldn’t really do much. I was only eating a couple of times a week.
‘I stopped being able to walk. I would take a couple of steps and have to stop. Then I couldn’t stop being sick.

Aimee Chapman lost four stone within four months on Mounjaro—but she never expected the devastating side effects
‘I was throwing up all the time and started throwing up blood. I was sick between 50 and 60 times.
‘I collapsed a couple of times. My husband came home and found me passed out on the hallway one night.
‘I was in a bit of denial about it being linked to the jab because I was fine up until now. I just thought I was poorly and it was some sort of virus.’
When she began experiencing chest pains, she went to Winchester Hospital’s A&E where doctors discovered a hole in her oesophagus—the tube connecting the back of the mouth to the stomach.
This was allowing air to fill the space around her heart and lungs.
Her blood pressure and potassium levels then began to plummet, forcing doctors to admit her to intensive care.
Ms Chapman was later rushed to Southampton General Hospital’s ICU, where doctors noticed her liver was ‘failing’, prompting them to consider an organ transplant.
‘They had said it was down to the weight-loss jab but they didn’t know why or how to fix it,’ she said.
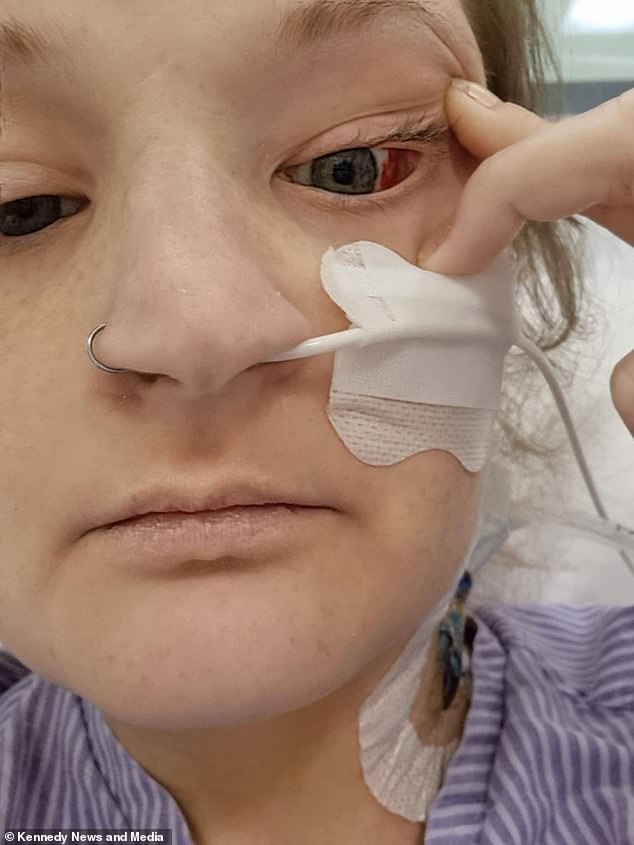
Medics found that her liver was failing and she’d developed a hole in her food pipe, according to the former waitress

Six months after she began injecting the drug, Ms Chapman noticed her hair began to fall out in significant chunks
‘I was terrified. It all happened so quickly and I hadn’t realised how serious it was until I was transferred and it sunk in that it was worse than I thought it was.
‘I was told the hole in the oesophagus can kill people and I may have needed a new liver. They said I could’ve died.’
Luckily, doctors were able to stabilise her condition within two weeks, and she was discharged from hospital.
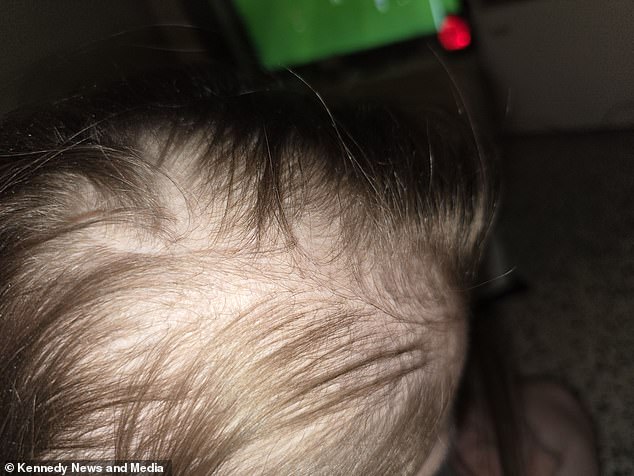
The ordeal was not over, however. A few months later, in September, Ms Chapman began to notice large clumps of her hair falling out.
Subsequent blood tests revealed the cause of the problem—a deficiency in the vitamin B12, which is essential for maintaining the supply of oxygen to the hair follicles.
A wealth of research has shown that any form of rapid weight loss, whether from medication or surgery, can dramatically increase the risk of nutritional deficiencies.
‘It kept happening and the handfuls kept getting bigger. I would be so sad,’ said Ms Chapman.
‘My hair was such a massive part of my identity and to cut it off just felt really traumatic. But I said to myself it was just hair, it’ll grow back. This feels like another result of the injections.’

The hair loss was said to be the result of a vitamin B12 deficiency, which has previously been linked to extreme weight loss over short periods of time
Ms Chapman regrets taking the drug and would recommend anyone considering it seeks advice from their GP as well as regular blood tests
While she doesn’t wish to ‘tell people what they can and can’t take’, she believes ‘more research’ is needed to confirm the safety of weight loss injections.
‘I regret ever taking them,’ she said.
‘I’d say to other people thinking about trying them, don’t do it online, go through your doctor who can give you blood tests and check how you’re doing.’
In September last year, medics from Kuwait reported the case of a 24 year-old teacher who began suffering from extreme vomiting, nausea and stomach pains after using Mounjaro—often referred to as the ‘King Kong’ of weight loss jabs.
Hospital tests revealed that the patient, who was obese and had a BMI of 34, had ‘alarming’ levels of liver enzymes, proteins that help break down bile and toxins.
This was a sign that her organ was failing and she was admitted to the ICU where she was given a plasma transfusion to flush out the toxins in her blood.
She was referred to a transplant team because if left untreated, she could have suffered full blown liver failure, and a transplant would have been her only chance of survival.
Writing in the European Journal of Case Reports, doctors from Kuwait who treated her said Mounjaro was ‘likely the susceptible cause.’
The case came a year after a 37 year-old in Seattle was treated for a similar liver injury after taking the same drug.
Experts believe liver damage may happen in rare cases due to the rapid reduction of fat in the liver that could kill healthy cells.
Meanwhile, patients have previously spoken of how Ozempic—which works similarly to Mounjaro—have triggered vomiting so violent it’s led to a hole in their food pipe.
Ozempic and Wegovy contain the active ingredient semaglutide. This mimics the hormone GLP-1 (glucagon-like peptide-1) which slows the movement of food through the digestive system, signalling to the body that it’s full.
Mounjar, meanwhile, use the active ingredient tirzepatide, which targets GLP-1 as well as the hormone glucose-dependent insulinotropic polypeptide (GIP), which has a similar hunger-surpressing effect.
The dual action is thought to accelerate weight loss, making it more effective than Ozempic.
In a statement, Eli Lilly, the drug firm that makes Mounjaro said: ‘Patient safety is Lilly’s top priority.
‘We are committed to continually monitoring, evaluating, and reporting safety information for all Lilly medicines. Mounjaro (tirzepatide) was approved based on extensive assessment of the benefits and risks of the medicine, and we provide information about the benefits and risks of all our medicines to regulators around the world to ensure the latest information is available for prescribers.
‘If anyone is experiencing side effects when taking any Lilly medicine, they should talk to their doctor or other healthcare professional.’











