When Susan McGowan died after just two injections of Mounjaro she’d bought from an online pharmacist, health officials rushed to reassure the public on the safety of the new generation of weight-loss jabs.
The death certificate for the 58-year-old nurse from North Lanarkshire, who died last September, listed acute pancreatitis – inflammation of the pancreas – as one of the immediate causes of death. Her use of Mounjaro (or tirzepatide) was recorded as ‘a contributing factor’.
It was the first death officially linked to the drug in the UK.
At the time, Dr Alison Cave, chief safety officer for the Medicines & Healthcare products Regulatory Agency (MHRA) – the UK body that vets drug safety – expressed sympathy for Susan’s family, but insisted: ‘On the basis of the current evidence, the benefits [of these drugs] outweigh the potential risks when used for the licensed indications.’
Just days after NHS England started offering Mounjaro to eligible obese people who need help to manage their weight, these weight-loss jabs are in the spotlight again following concerns about possible links to pancreatitis.
The MHRA has revealed it’s received more than 560 reports of acute pancreatitis in patients taking GLP-1 injections since they were first approved in the UK; liraglutide (brand name Saxenda) was approved in 2017, semaglutide (Wegovy and Ozempic) in 2023, and tirzepatide last year.
Ten cases have been fatal – five of them related to tirzepatide.
The pancreas is a pear-shaped organ behind the stomach that produces digestive enzymes and insulin.

Susan McGowan died after just two injections of Mounjaro she’d bought from an online pharmacist
Acute pancreatitis is a painful condition which occurs over a short period of time when the pancreas becomes inflamed.
Most cases improve without treatment within about a week and have no further problems. But some people can go on to develop serious complications including necrosis, where the tissue of the pancreas starts to die. This can lead to infection, sepsis and organ failure.
The main symptom of pancreatitis is severe pain in the stomach that radiates to the back and does not go away; it can also cause fever and vomiting.
Just two weeks after she was prescribed Mounjaro, Sarah Miller, 54, an office administration assistant from Caerphilly, started to experience a ‘niggling’ pain on her left side just under her ribcage.
After the menopause her weight had ballooned to 18st (she’s 5ft 9in), and she’d developed type 2 diabetes – she also has the autoimmune condition lupus, which can make exercise difficult.
‘The nurse said Mounjaro would help me lose weight and might even put my diabetes into remission,’ says Sarah. She was started on a low 2.5mg dose once a week.
‘At first all was well: I noticed decreased appetite and was excited as I started losing weight.
‘But two weeks in, I felt like I’d pulled a muscle. As the hours passed the pain got worse. I tried paracetamol; it didn’t touch it. Then I tried ibuprofen, then the two together. Nothing helped.’

The MHRA has revealed it’s received more than 560 reports of acute pancreatitis in patients taking GLP-1 injections since they were first approved in the UK
Then after about two weeks, one night she developed ‘horrendous acid reflux’.
‘I woke up coughing and spluttering and couldn’t catch my breath,’ she recalls. ‘I called 111 and they gave me the details of the out-of-hours doctor, but by morning the acid had gone. However, the pain was now constant and really ruining my daily life.
‘Coincidentally, that day I saw a news report about a woman who had taken Mounjaro and developed pancreatitis and died. I Googled the symptoms then immediately stopped taking Mounjaro – and booked an urgent appointment with my GP.
‘Within days of stopping the medication, the pain went.’
The MHRA is now keen to get a clearer idea of the scale of the problem by encouraging medics and patients who’ve been hospitalised to report this side-effect via its Yellow Card scheme – an online reporting system that allows patients and doctors to alert the MHRA to suspected adverse drug reactions.
So should the estimated 1.5 million people in the UK who already use weight-loss jabs be worried?
Based on the data we do have, acute pancreatitis is a rare side-effect – tirzepatide is known to cause it in just 0.39 per cent of users. Why it happens is still up for debate.
‘One theory is GLP-1 agonists may contribute to acute pancreatitis by over-stimulating GLP-1 receptors in duct cells (in the pancreas) which secrete digestive enzymes into the small intestine,’ says Alexander Miras, a clinical professor of medicine at the Ulster University and the Western Health & Social Care Trust.
‘If these over-produce enzymes it can cause widespread inflammation and tissue damage if they leak inside the pancreas.’
‘One theory is GLP-1 agonists may contribute to acute pancreatitis by over-stimulating GLP-1 receptors in duct cells,’ says Alexander Miras
He points to the fact that the GLP-1 class of drugs have been used for 18 years successfully to help people with diabetes without any major alerts over the risks of pancreatitis.
There is no physiological reason why the same class of drugs should start to cause more problems in people using them to lose weight than those using them to manage diabetes, he adds.
‘We know from multiple trials that less than three in 1,000 people on these drugs develop acute pancreatitis, and often this may be down to the fact that they have other health problems that make them more susceptible to this problem,’ Professor Miras told Good Health.
‘We need to be cautious and keep an open mind – but until the MHRA investigations have concluded, we don’t want to unnecessarily alarm patients by telling them the medications are potentially lethal in very rare cases.’
But as more people take the jabs – which work by mimicking a hormone, GLP-1, that’s released in the gut when we eat and acts on receptors around the body, including the brain – concerns are growing that more people could end up suffering complications.
‘The problem is that we don’t know enough to say for sure right now,’ says Christian Macutkiewicz, a consultant surgeon specialising in problems with the liver, pancreas and gallbladder at Manchester Royal Infirmary.
‘We have longer-term data about GLP-1 agonists when they are used to treat diabetes, but not when they are used for weight loss alone.’
Is it a good idea to switch from Mounjaro to other weight-loss jabs? Professor Miras says that all GLP-1 medications have similar risk profiles but some are prescribed more often than others.
‘Mounjaro is one of the most widely prescribed drugs in this class, so this may be one of the reasons more people develop this uncommon side-effect.’
Another reason for not switching between GLP-1 medications is that this may actually cause more harm than good, according to 2024 research in the Cureus Journal of Medical Science.
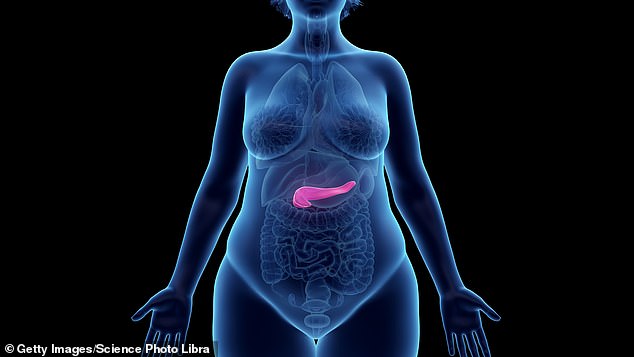
Acute pancreatitis is a painful condition which occurs over a short period of time when the pancreas (in red) becomes inflamed
Researchers at the University of Florida found that switching actually made it more likely that someone would develop acute pancreatitis – ‘especially if appropriate dose titration protocols are not followed’. In other words, the side-effects may be worsened if the dose is incorrectly calibrated and is too high.
One way to help reduce the risk of pancreatitis may be not to give these drugs to patients who are more prone to developing the condition – such as those who have already had pancreatitis and are at higher risk of it returning, or gallstones (a major cause of pancreatitis).
Researchers are also examining whether those affected have a genetic trait that leaves them at greater risk of side-effects from GLP-1 agonist drugs.
Professor Matt Brown, chief scientific officer of Genomics England, is leading a team that will analyse genetic information from people who suffer acute pancreatitis linked to their fat jab use and submit a saliva sample to the Yellow Card Biobank – a database of DNA used to analyse why some people are more susceptible to drug reactions than others.
He said: ‘We believe there is real potential to minimise these, with many adverse reactions having a genetic cause.’
In the meantime, Professor Miras points out that risks always need to be carefully weighed against benefits.
‘Taking these jabs may be reasonable if you are living with obesity or diabetes and their complications, because they will improve your overall health.
‘However, people who are using these drugs for cosmetic reasons, just to be thinner, should be warned that there are risks that come with them and the consequences could be critical illness.’
A spokesperson for Novo Nordisk, which makes Ozempic and Wegovy, told Good Health: ‘Patient safety is of the utmost importance. The known risks and benefits of GLP-1 medicines are described in the summary of product characteristics.
‘In the clinical trials conducted by Novo Nordisk, pancreatitis was reported as an adverse event in 0.1 per cent of Wegovy-treated patients and less than 0.1 per cent in patients given a placebo.
‘We recommend that patients take these medications only for their approved indications and under the strict supervision of a healthcare professional, who can advise on potential side-effects.’
A spokesperson for Eli Lilly, the maker of Mounjaro, said: ‘We take reports regarding patient safety seriously and actively monitor, evaluate and report safety information for all our medicines. Adverse events should be reported under the MHRA’s Yellow Card scheme, but may be caused by other factors, including pre-existing conditions.
‘The Mounjaro (tirzepatide) patient information leaflet warns that inflamed pancreas (acute pancreatitis) is an uncommon side-effect.’
Meanwhile, Sarah is now managing her diabetes with standard medication and a low-fat diet.
‘My weight now is about half a stone heavier – but my diabetes is under control,’ she says.
‘I think Mounjaro is a wonder drug when it works and I did lose 3 lb in those two weeks – but I think too many of us are taking it blindly, without cross-referencing other conditions we might have.’
Additional reporting by Julie Cook











