Heartbroken pet owners are fighting for a £100-a-month ‘wonder drug’ for dogs with arthritis to come with a warning after a spate of deaths linked to the medicine.
Librela, also known as Solensia for cats, is a monthly injection to help relieve the pain associated with osteoarthritis in dogs and is only available on prescription.
It can be purchased online for around £100 for two vials.
Five-star reviews call the medicine ‘liquid gold’ and a ‘game changer’ for dogs who suffer from joint pain.
Many describe their pets being able to run and play like they did as puppies after years of suffering but some owners have found their pets suffered serious adverse effects.
Rebecca Hudson turned to Librela in July 2021 when her vet recommended it to treat her dog Sam’s elbow dysplasia.
She was told it was an ‘amazing new drug’ and claims she was not warned of any side effects.
After three years of having injections every six months Rebecca noticed eight-year-old Sam had started walking with his back leg bowing and his foot rolling underneath him.

Librela injections can be purchased online with a prescription for around £100 for two vials

Rebecca Hudson (pictured) turned to Librela in July 2021 when her vet recommended it to treat her dog Sam’s elbow dysplasia

After three years of having injections every six months and everything going well, Rebecca noticed eight-year-old Sam had started walking with his back leg bowing and his foot rolling underneath him
It took visits to three different vets before Sam’s problems were taken seriously in September last year.
Rebecca told MailOnline: ‘The vet was extremely concerned that he could move Sam’s ankle joint as far side to side as he could front to back and recommended X-rays.
‘The X-rays showed the joint was destroyed and he referred us to the specialist Orthopaedic Hospital.
‘The vet said his joint was completely destroyed and they did not know how he was able to walk on it.
‘The bones were so crushed up together and he was obviously in a lot of pain.’
Sam was kept in hospital for three days while he underwent a six-hour surgery to fuse his bones.
He stopped taking Librela shortly before his operation.
‘I keep crying because I feel I have poisoned him,’ Rebecca said.
‘The surgery was very invasive and he was in a terrible state afterwards.
‘He became incontinent and was peeing all over the house, which he had never done before.
‘It became so severe that he had to wear a belly band to save the soft furnishings.
‘While Sam’s surgeon said he cannot say for certain Librela caused this rapid onset osteoarthritis, he could not find another possible cause.’
Since Sam had his last Librela injection in September Rebecca said she has seen massive changes in his behaviour which she attributes to stopping the drug.
‘His leg is recovering and although he can’t bend his leg, he is adapting,’ she said.
‘He is going to hydrotherapy and can swim well again, which is what he always enjoyed doing.
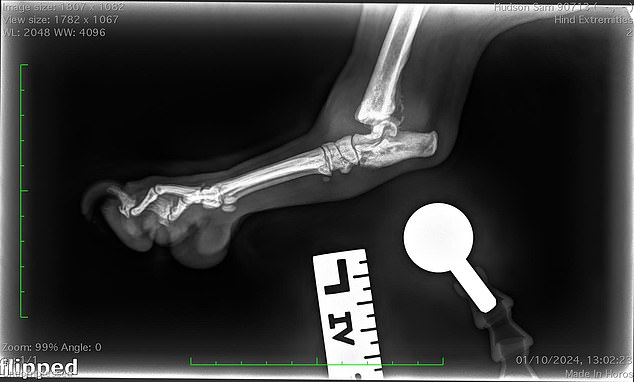
Rebecca said: ‘The vet said his joint was completely destroyed and they did not know how he was able to walk on it.’ The above image is an X-ray of his joint
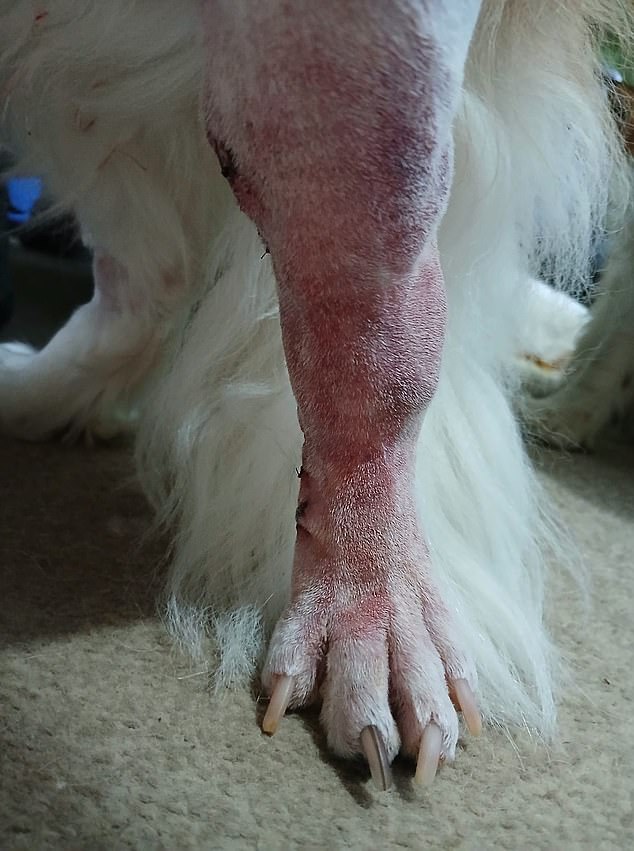
‘This major operation on Sam’s leg has been shocking to us,’ Rebecca said. Sam’s leg is shown post-operation
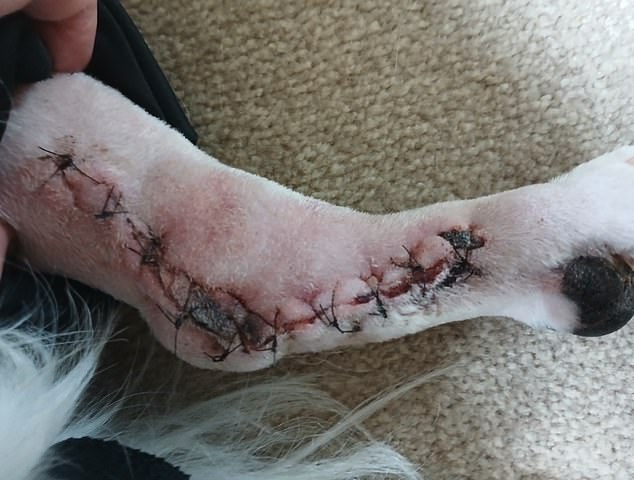
Sam was kept in the vets for three days while he underwent a six-hour surgery to fuse his bones. The above image shows his stitches
‘Sam is just a much more relaxed and content dog. He is enjoying his cuddles now whereas before he would never stay still enough to let you get near him.’
The cost of getting Sam well again was enormous with surgeries and vet bills totalling more than £10k last year.
‘Sam will never have another Librela injection,’ Rebecca said.
‘This major operation on his leg has been shocking to us.
‘The fact that something given to treat elbow dysplasia has destroyed his hock joint is so counter intuitive I’m finding it hard to get my head round.’
Librela, manufactured by animal health company Zoetis, is relatively new to the market and was only approved for use in the European Union in November 2020 and in the US in 2023.
But shortly after its release in the US the Food and Drug Administration (FDA) issued a warning with side effects of the drug includes seizures, lameness, loss of muscle control and in some cases, death, including euthanasia.
This came after more than 3,600 cases of side effects were reported to them between the whole of 2023 and March 2024.
Despite efforts by campaigners, the same warning has not been given by the Veterinary Medicines Directorate (VMD), meaning many pet owners are unaware of the potential side effects of the drug.
In December 2024 the VMD added diarrhoea, vomiting, ataxia, urinary incontinence, anorexia and lethargy as rare side effects of the medication as well as seizures in very rare cases.
Prior to this update the possible side effects listed were injection site reaction, thirst, excessive irination, hypersensitivity reaction, immune-mediated haemolytic anaemia and immune-mediated thrombocytopenia, which causes an increased risk of bleeding.

Rebecca said: ‘Sam is just a much more relaxed and content dog. He is enjoying his cuddles now whereas before he would never stay still enough to let you get near him’

Tina Doo lost her 12-year-old German Shephard Snowy – pictured – last November within two weeks of her taking her second Librela shot

Snowy was prescribed Librela after she began showing symptoms of degenerative myelopathy, a disease that affects the spinal cord in dogs
Tina Doo lost her 12-year-old German Shephard Snowy last November within two weeks of her taking her second Librela shot.
She told MailOnline: ‘The progression was so quick. She went from a heathy and happy dog to not being able to hold herself up and having no interest in anything.’
Snowy was prescribed Librela after she began showing symptoms of degenerative myelopathy, a disease that affects the spinal cord in dogs, causing progressive muscle weakness and loss of coordination.
Librela was offered as an alternative to oral pain relief drugs which Tina struggled to get Snowy to take.
Tina said: ‘When I was told by the vet that Snowy could have a monthly injection which I could administer myself, I thought it was great.
‘I was told there were no side effects and was able to order it online and keep it in my fridge.
‘Knowing what I know now, I do not think people should be allowed to inject it at home. There is the potential for somebody to mistakenly inject themselves which could do real damage.’
Tina noticed Snowy was not able to walk as far as she used to and her legs began to knuckle every few steps.
Snowy had her second, and last, injection on November 4, 2024, five weeks after her first.
Tina said: ‘After a few days we noticed she was started to drink more water and she would tire more easily on walks.
‘She no longer asked for her walks and her appetite changed. She went from having a healthy appetite to only eating chicken.’
After Snowy had a mini seizure in November Tina and her partner realised the only thing which had changed in their dog’s life in the past months was Librela.
They took Snowy to the vets and were told after a physical examination that she was fine.
The vet said Snowy’s symptoms were unlikely side effects of Librela – which Tina had began Googling and learning more about – but they decided to take her off the drug anyway.
By November 20 she was refusing to eat anything and two days later she could not walk at all.

After Snowy had a mini seizure in November Tina and her partner realised the only thing which had changed in their dog’s life in the past months was Librela
‘It got to the point where she couldn’t walk at all and we had to carry her outside to do her wees because she couldn’t hold herself up,’ she said.
Her nose and gums had also begun bleeding.
On November 23 Tina and her partner reluctantly made the decision to put Snowy down.
She said: ‘That day she was completely flat out and wouldn’t sit up.
‘We couldn’t ask her to carry on like this.’
While Tina says she will never know for certain what caused Snowy’s symptoms, she says her experience is matched by many other pet owners who have given Librela to their dogs.
‘People need to understand taking Librela is playing Russian Roulette with your dog’s life.,’ she said.
‘Libela is often given to older dogs and when they die it’s put down to old age.
‘A lot of people don’t realise Librela is what is killing them.
‘There are dogs that tolerate it and for them and it gives them a new lease of life.
‘But people need to be able to give informed consent, knowing that there will be a chance their dog will die.
‘It should be used in cases where euthanasia is the last resort.’
A Zoetis spokesperson said: ‘Since launching four years ago Librela has been used effectively with millions of dogs around the world, helping dogs suffering from osteoarthritis pain live with less pain and greater mobility.
‘With more than 25 million doses distributed globally, no single sign reported as an adverse event is classified as more than rare, according to the European Medicines Agency (EMA) categorization.
‘We remain confident in the safety and effectiveness of Librela for controlling osteoarthritis pain in dogs, when used according to the label, and in the science, safety and regulatory rigor behind this product.
‘As the world’s leading animal health company, we understand that when any dog experiences an adverse event, these statistics are a small consolation. All medicines come with some potential risks. Pet owners should weigh the benefits of using any product against the potential risks in consultation with their veterinarian.
‘When Zoetis receives reports of potential adverse events from pet owners or veterinarians, we take very seriously our obligation to gather as much relevant information as possible. We do this so we can ensure the ongoing safety of our products and so that we can report these events to the appropriate regulatory agency.’
A VMD Spokesperson said: ‘We would always advise the public to seek advice from their veterinary professional before providing medicine to their animals.
‘As with all veterinary medicines in this country, we have continuously monitored Librela since it was first authorised. Data shows fewer than 15 animals have experienced side effects for every 10,000 doses of Librela sold.
‘We take any reports of adverse side effects very seriously and will take action where appropriate, such as ensuring additional warnings are added to packaging or changing the way a product is used.’







