The first needle-free treatment for life-threatening allergic reactions will be made available in the UK as a nasal spray.
The medicines regulator has approved the drug EURneffy for emergency use in a move welcomed by allergy campaigners.
They say it provides an ‘easier and more accessible’ alternative to injectable epipens, which are currently used to treat severe reactions, known as anaphylaxis.
Companies are required by law to clearly tell customers if their food contains any of 14 specified allergens, which have the potential to kill.
These include nuts, crustaceans, eggs, fish, milk, mustard and sesame. Some people also suffer fatal reactions to insect stings and medicines.
Tanya Ednan-Laperouse, whose daughter Natasha died in 2016 after eating a Pret baguette containing sesame, said: ‘The number of people experiencing anaphylaxis triggered by food has increased dramatically over the last 20 years.
‘But we know that some people are reluctant to use the current adrenaline auto-injectors in the event of anaphylaxis due to a fear of needles and hurting someone.
‘This can delay administering adrenaline, and in a food allergy emergency every second counts.
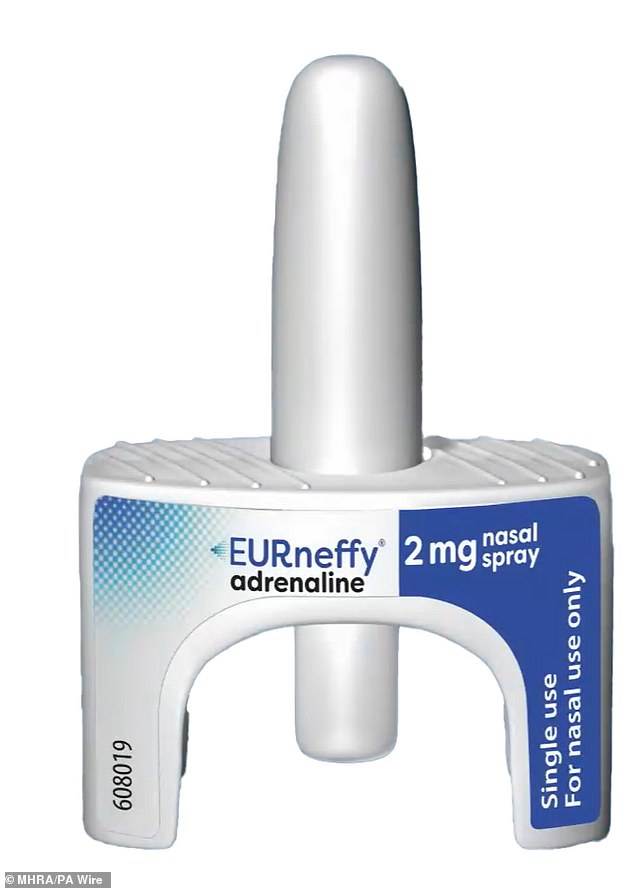
The medicines regulator has approved the drug EURneffy for the emergency treatment of severe allergic reactions
‘The nasal spray will be an easier and more accessible way of administering this life-saving medication, and is great news for people living with food allergies.’
Mrs Ednan-Laperouse, who founded food allergy charity the Natasha Allergy Research Foundation in her daughter’s name, added: ‘Spare supplies of these nasal adrenaline devices should now be another option available to schools.’
The Food Standards Agency says up to 2 per cent of adults and 8 per cent of children in the UK live with a food allergy.
Anaphylaxis is a sudden and life-threatening allergic reaction that can cause a drop in blood pressure and breathing difficulties.
More than 7,000 birth certificates a year in the mention anaphylactic shock, according to the Office for National Statistics.
EURneffy, which contains adrenaline, is intended for use in adults and children who weigh 30kg (66lb) or more, and can be used even if people have colds or blocked noses.
It is a single dose nasal spray that delivers its entire contents (2mg) upon activation.
People are reminded the plunger should not be pressed before inserting the product into the nostril, otherwise the single dose will be lost.
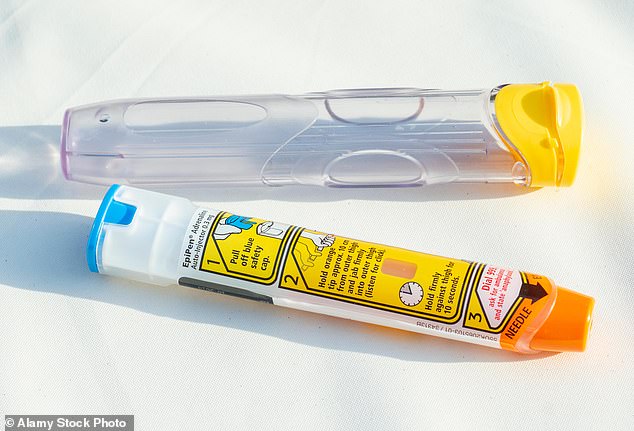
Allergic reactions are currently treated with adrenaline auto-injectors known as an epipen
Julian Beach, from the Medicines and Healthcare products Regulatory Agency, said: ‘Patient safety is our top priority, which is why we’re pleased to approve the first needle-free nasal spray formulation of adrenaline for the emergency treatment of anaphylaxis in the UK.
‘Until now, adrenaline for self-administration has only been available via auto-injectors.
‘While this represents an important new option, adrenaline auto-injectors remain a vital and potentially life-saving treatment, giving people experiencing anaphylaxis valuable time before emergency help arrives.
‘We continue to encourage everyone at risk of severe allergic reactions, and those around them, to familiarise themselves with how to respond in an emergency.
‘Resources and guidance are available on the MHRA website to help people be prepared.’
The MHRA said patients should always carry two nasal sprays with them in case a second dose is needed, and tell family and friends where it is.
A spokeswoman for the drug firm behind the spray, ALK, said: ‘The market launch in the UK is expected within the coming months once market access negotiations are completed.’

Natasha Ednan-Laperouse died in 2016 after eating a Pret baguette containing sesame
ALK is having ongoing discussions to agree a price with the Department of Health and Social Care.
Once an NHS list price has been agreed and the product is available, doctors will be able to prescribe the nasal spray.












